Pyrolysis is a thermochemical decomposition of organic material at elevated temperatures without the participation of oxygen. It involves the simultaneous change of chemical composition and physical phase and is irreversible. The word is coined from the Greek-derived phrase pyr “fire” and lysis “separating.“ In fact, you can think of analytical pyrolysis as breaking apart or manipulating organic molecules in a controlled and predictable manner using the precise use of heat.
Pyrolysis GCMS is a powerful and straightforward technique comprised of a Frontier Pyrolyzer coupled with a gas chromatography-mass spectrometer system. The sufficient amount of heat is used to break down the organic bonds of polymers into smaller and stable molecules referred to as pyrolyzates. The pyrolyzates formed and their relative intensities provide insight into the nature of the original material. The figure below demonstrates the degradation mechanism of polyethylene when the sufficient amount of heat is applied in the presence of an inert gas, such as Helium compared to applying the heat in the presence of oxygen.
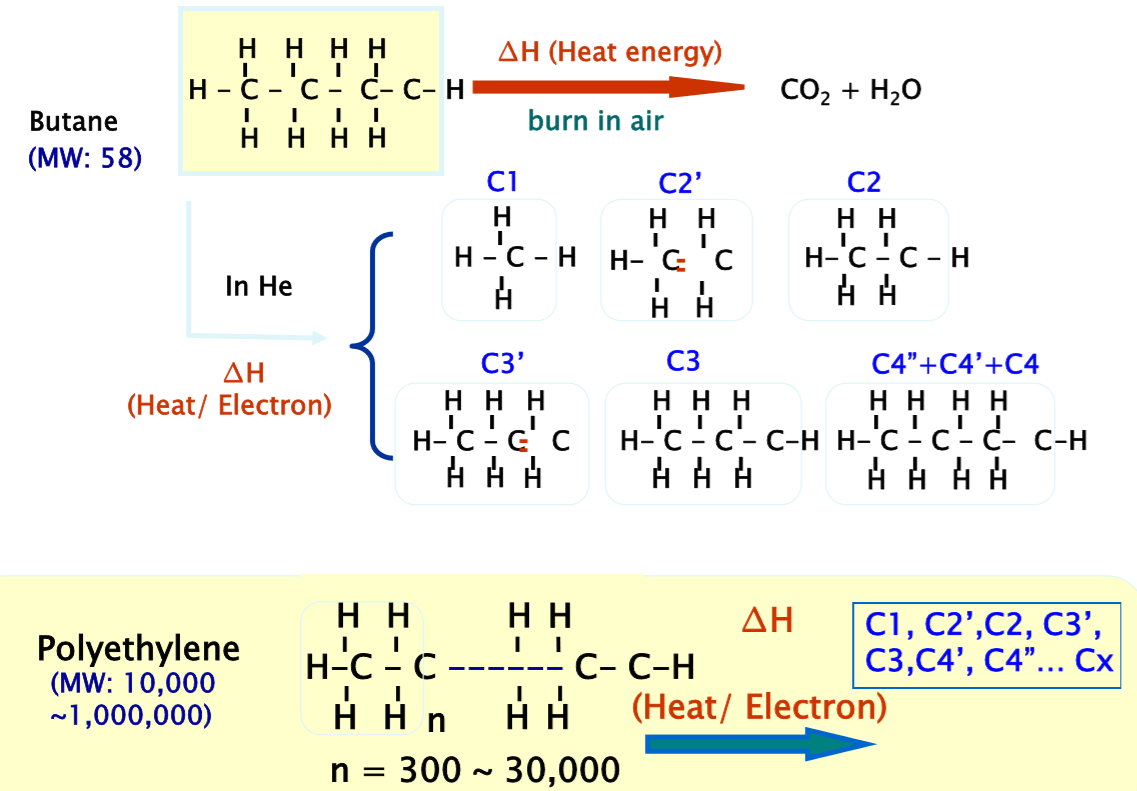
As shown in the figure, butane breaks down into different alkane and alkene pyrolyzates randomly when the analysis is performed in the presence of Helium. The pyrolyztes are identified using F-Search Pyrolyzate-MS library to determine the polymer(s) present in a sample. However, when oxygen is present, carbon dioxide and water are formed as the result of the heat.
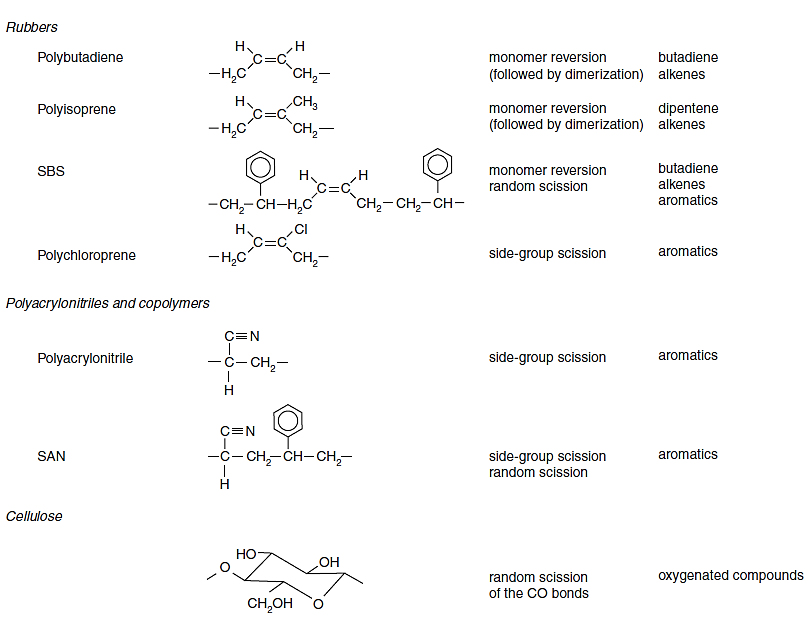
The organic bonds of polymers break down in different degradation mechanisms depending on the structure of the polymer and the energy needed to break down the bonds. The primary polymer degradation mechanisms are described here:
- Random Scission: C-C bonds break to produce fragment patterns of increasing oligomer sizes. Example: Polyolefins (polyethylene, polypropylene, polybutylene, etc.)
- Depolymerization: Polymer thermally degrades into monomeric units. Example: Polystyrene shows monomer (S), dimer (SS) and trimer (SSS).
- Side Group Elimination: Side groups (i.e., Cl) attached to the side of a polymer chain break before C bonds. Cl removes H from polymer chain which results in unsaturated polyenes + HCl. These polyenes form aromatic compounds. Example: PVC pyrolyzates contain single aromatic rings (BTEX), double rings (i.e., naphthalene), triple rings (i.e., anthracene), and the HCl peak.
The table below shows more examples of degradation mechanism for different polymers. Note that some polymers might have a combination of two degradation mechanisms.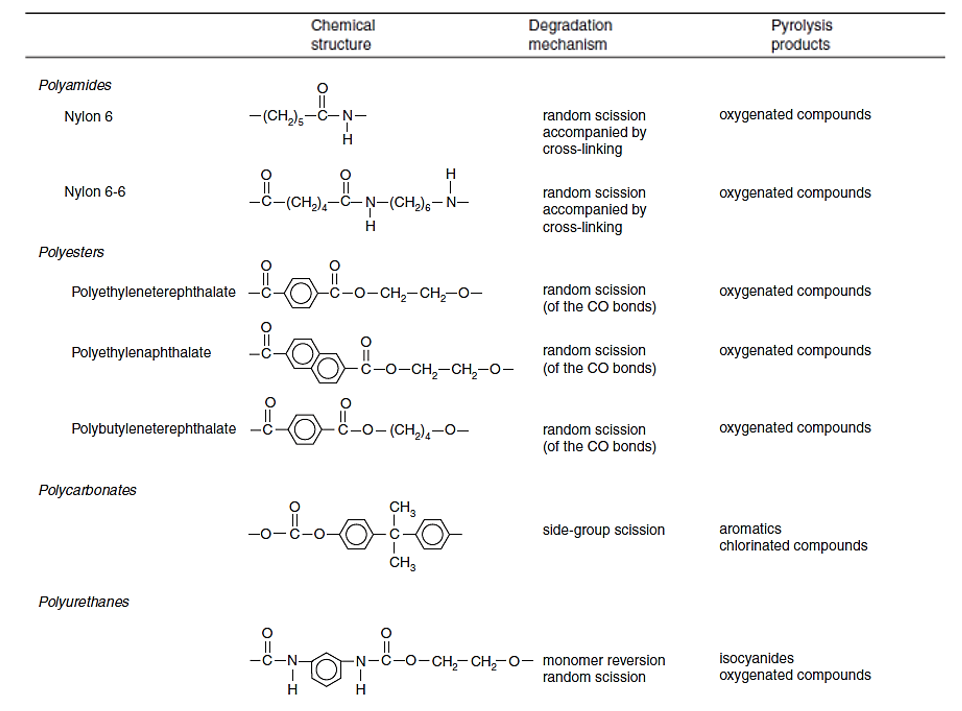
Reference: E. Stauffer, Sources of Interference in Fire Debris Analysis, in Fire Investigation (Ed.: N. Nic Daeid), CRC Press, Boca Raton (2004). http://dx.doi.org/10.1201/9780203646953.ch7
To learn more about Pyrolysis GCMS technique and how it can add value to your analytical laboratories, simply connect with us. You can request technical application notes performed by the Pyrolysis GCMS technique related to your industry and research.
